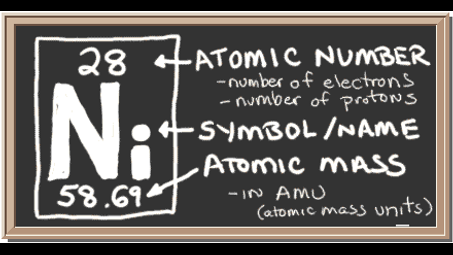
28 electrons
Nickel atoms have 28 electrons and the shell structure is 2.8.
What else can I help you with?
How many core electrons does He have?
Helium has 2 core electrons. This is because it has 2 electrons in the innermost energy level (K shell), which are considered core electrons.
How many core electrons In hydrogen?
Hydrogen has one core electron. Core electrons are those in the inner energy levels of an atom and are not involved in chemical bonding.
How many core electrons does argon have?
Argon has 18 core electrons. This is because the atomic number of argon is 18, and the number of core electrons is equal to the number of electrons in the nearest noble gas configuration, which in this case is neon (10 core electrons), plus the number of electrons in the next energy level, which is 8 for argon.
How many electrons does Ni 3 plus have?
Nickel is a chemical metal element. There are 28 electrons in a single atom.
How many core electrons does Br have?
Bromine (Br) has 18 core electrons. This can be determined by subtracting the number of valence electrons (7 in the case of bromine) from the total number of electrons in a neutral atom, which is 35 for bromine.
How many core electrons does germanium?
Germanium has 36 core electrons. Core electrons are the inner electrons that are not involved in chemical bonding.
How many core electrons are in Ion?
Oxygen has 6 core electrons.
How many core electrons in sn?
Tin has 46 core electrons.
How many core electrons does germanium have?
Germanium has 18 core electrons. Core electrons are the inner electrons of an atom that are not involved in chemical bonding.
How many core oxygen electrons?
Oxygen as 2 core electrons and 6 valence electrons.
How many core electrons does carbon have?
Carbon has 2 core electrons. Core electrons are the inner electrons of an atom that are not involved in chemical bonding.
How many core electrons are in oxygen ion?
Oxygen has 6 core electrons.
How many core electrons in argon?
Argon has 18 core electrons. This is because it has 18 electrons in total, and the core electrons are the innermost electrons that are not involved in chemical bonding.
How many core electrons does He have?
Helium has 2 core electrons. This is because it has 2 electrons in the innermost energy level (K shell), which are considered core electrons.
How many core electrons in a silicon?
Silicon has a total of 10 core electrons and 4 valence electrons.
How many core electrons does nitrogen have?
Nitrogen has 2 core electrons. Core electrons are those that are found in the inner energy levels and are not involved in chemical bonding.
How many core electrons does tungsten have?
Tungsten has 46 core electrons. Core electrons are the electrons found in all elements that are not valence electrons. Tungsten has an atomic number of 74, so it has 74 electrons in total.
